Scarring is a common issue for patients with acne. Typical treatment options include topical, surgical and laser options. Acne scars were top of mind at ASLMS 2024 with several abstracts exploring innovative ways to treat acne scarring.
Related: Why Exosome Combination Therapy is the New Gold Standard for Treating Acne Scars
Nasal Ice Pick Scar: Stephen A. Wirya, M.D. - Dr. Ratna Dermatology Center
This study analyzed the results of two therapeutic options for addressing nasal ice pick scars. These scars are small and deep, often resembling the shape of an ice pick. Icepick scars are notoriously known as the hardest acne scars to treat. The two leading therapeutic options for addressing these scars are the TCA Chemical Reconstruction of Skin Scars (CROSS) technique, involving trichloroacetic acid (TCA) application to resurface the skin, and punch excision, which uses a circular tool to remove scar tissue and suture the wound.
The study found that while both methods show promise, TCA CROSS may be limited by the nose's curvature, potentially affecting nearby healthy skin, and punch excision can be effective for individual ice pick scars but less suitable for closely grouped scars, complicated by the nose's small size and the risk of anatomical distortion.
The approach involved the targeted treatment of individual nasal ice pick scars using a fractional ablative CO2 laser while sparing the rest of the face. Follow-ups at week 16 revealed that four patients had clinical improvement of 76-100%, one had clinical improvement of 51-75% and none had minimal or no improvements. No severe adverse effects were reported.
The proposed approach introduces an innovative method for addressing nasal ice pick scars, involving the targeted application of a fractional ablative carbon dioxide (CO2) laser to treat individual scars.
Standardized Outcome Reporting for Acne Scarring: Misha Haq, BS - Northwestern University
This study aimed to identify all outcomes that have been utilized in randomized controlled trials (RCTs) of acne scarring, with the intention of developing a core outcome set for acne scarring.
Of the 50 articles analyzed, 24 (48%) evaluated laser-based treatments. 50 unique outcomes were identified from the systematic review. Erythema was the most measured adverse event outcome (35 studies, 70%).
Additionally, edema (28 studies, 56%), post-inflammatory hyperpigmentation/hypopigmentation (26 studies, 52%), pain (23 studies, 46%), and scab/crust formation (16 studies, 32%) were commonly measured adverse events.
Physician global assessment of scars was evaluated in 46 of the 50 studies (92%), followed by patient satisfaction (30 studies, 60%) and histopathological assessment of the scar (19 studies, 38%). Quality of life was only measured in 5 of the 50 studies (10%).
As determined in the systematic review, gaps remain in the standardization of outcome reporting across clinical trials of acne scarring. Utilizing the outcomes outlined in this review, a core outcome set can be developed to address these concerns regarding outcome heterogeneity
High-frequency Ultrasound: Julian Henke, BS - Emory University, School of Medicine
High-frequency ultrasound (HFUS) is a non-invasive, cost-effective imaging modality that allows for a high-quality resolution of skin and structures, including the epidermis, dermis, subcutis and superficial muscle HFUS has been used to evaluate the progression of various scar treatments including drug, laser and lipofilling therapies. The study provides a unique insight into the clinical identification and differentiation of scar formation resulting from trauma to surgery through the lens of HFUS imaging.
Researchers retrospectively assessed HFUS images of scars taken from a collection of 66 lesions imaged in a single academic institution-affiliated dermatology outpatient clinic in Atlanta, Georgia, through an IRB-approved study. A 50 MHz HFUS device (Longport, Inc. EPISCAN) was used with a rate of one scan per frame per second, a 15 mm scan length and a scan depth of 3.8mm. They compiled 22 scars into three clinical subtypes: surgical, traumatic and keloid.
HFUS was found to be a promising non-invasive method for scar evaluation that provides physical information for laser treatment planning and follow-up. Assessing scar thickness before treatment gives HFUS potential to improve laser treatment outcomes of scars. For traumatic scars of varied depths presented in this case series, HFUS can map regions of varied scar thickness. Clinicians can therefore tailor scar treatment by adjusting laser penetration depth based on scar depth location. Future research is needed to investigate optimizing laser scar treatment based on depth and structure.
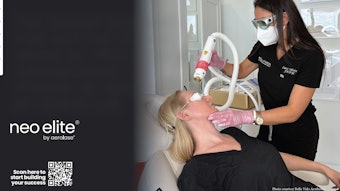
650-microsecond 1064NM:YAG Laser for Acne Management: Christine C. Dierickx, M.D.
This study assessed the efficacy, patient satisfaction and dermatologist perspectives on using the a 650-microsecond pulsed 1064nm Nd:YAG laser for acne treatment. Data was gathered from a survey conducted among 50 experienced users, primarily dermatologists. The survey entailed 26 questions detailing the clinical utility, efficacy, patient satisfaction and post-treatment outcomes of the Aerolase Neo Elite across diverse patient demographics and acne severities. Answers were analyzed using the Pearson chi-square test, Wilcoxon signed rank test, and Friedman’s test, reporting median, minimum, and maximum values.
The study found that 90% treated 50-100+ acne patients with the laser; 48% treated more than 200 patients. Most treated moderate acne most frequently, followed by severe and mild acne. There was a significant lesion count reduction across all severity levels, with respondents reporting at least 60-80% reduction for mild, moderate and severe acne. It demonstrated notable satisfaction levels, with more than 30% of respondents reporting greater than 80% satisfaction across all acne severities.
Comparison of FDA-cleared Devices for In-office Acne Treatment: Sara Osborne, BS - University of Minnesota Twin Cities School of Medicine
This study identified FDA-cleared devices approved for the treatment of acne vulgaris since 2022 and then reviewed the FDA database and contacted the device company to elucidate clinical evidence for each device. Each device company was contacted for available clinical data; devices were excluded if their manufacturers did not provide clinical studies.
The FDA database search yielded 105 devices; 12 were indicated for acne. Of the twelve device companies, three responded with clinical data. Eight studies were reviewed (six prospective open-label studies, one randomized control trial and one case report). All three devices had evidence of 90% or higher reduction in inflammatory lesions.
The most common adverse events were erythema, edema and mild pain during treatment; no permanent serious adverse events were reported in the reviewed studies. Some studies also tracked additional benefits beyond acne reduction, including histopathological improvements, sebum reduction, patient satisfaction, and long-term sustained acne reduction.
Synchronous Ultrasound Parallel Beam Technology to Improve Acne Scars: Arielle NB Kauvar, M.D. - New York Laser & Skin Care
Synchronous ultrasound parallel beam technology safely coagulates the mid-dermis to maximize neocollagenesis and neoelastogenesis, while providing feedback-controlled energy deposition and integrated skin cooling. The technology utilizes multiple high-frequency, low-divergent ultrasound beams that simultaneously deliver a high-density thermal dose to the mid-dermis centered at 1.5mm depth, increasing tissue temperatures to 60-70 degrees C and inducing tissue remodeling.
This clinical study evaluated the efficacy and safety of this technology on the appearance of acne scars. In the study, 67 subjects underwent three treatments (1-3 weeks apart) and were followed for three months after the last treatment. Three independent masked reviewers, who were not involved in the study, were asked to identify the pre- and post-treatment images, which were presented to them in a randomized fashion, and (2) to rank the acne scar severity (using a 4-point scale: 0=absent, 1=mild, 2=moderate. 3=severe). Anticipated tissue responses and adverse event occurrences were recorded throughout the study. Subjects ranked their discomfort during the treatments.
Following three treatment sessions, 97% of treated subjects showed improvement in acne scar appearance, as assessed by the correct identification of the pre- and post-treatment photographs by at least 2 of 3 blinded evaluators. There was a mean improvement of 1.05±0.53 units in the acne scar severity scale, reflecting an improvement of 46% from baseline scores.
Subjects were highly satisfied, with 88% reporting improvement in the appearance of their acne scars. There were no device-related adverse events. Anticipated tissue responses were erythema and edema, which resolved spontaneously after treatment. Most subjects reported none to moderate levels of pain during treatment and no discomfort afterward.
Picosecond Alexandrite Laser for Acne Scars on Darker Skin: Paul W. Wallace, M.D., MPA - Wallace Skin and Body Institute
This study explored how the picosecond Alexandrite laser with the focus lens array has demonstrated great clinical efficacy on acne scars in Fitzpatrick Skin Types I-IV, but additional data is needed to demonstrate its safety on Types V-VI.
The study analyzed 15 African American subjects that were 22-48 years old, 75% of the subjects were female, and 25% were male. All subjects had a Fitzpatrick Skin Type of V-VI, and the Melanin Index (MI) of the treatment area ranged from 75-87, with an average of 80.3 +/- 5.5. All subjects had been treated with a 755nm wavelength.
There was an average of 1583+/- 1145 pulses delivered per treatment (range of 243-4037 pulses), and a repetition rate of 10 Hz was used for all treatments. Pain scores were collected throughout treatments and followups were conducted.
During treatment, the average pain score reported by subjects was 3.7 +/- 2.4. The most commonly reported adverse events for this study are edema (n=6), erythema (n=5), and pain/tenderness (n=4). Based on reported start and end dates of adverse events thus far, edema lasted up to two days post-treatment, erythema lasted up to one-day post-treatment and pain/tenderness lasted a maximum of one day. The average duration of these adverse events across subjects was zero to one day. All adverse events have been mild, except for one moderate instance of edema that resolved within one day. There was no reported downtime from work or planned activities.