The U.S. Food and Drug Administration issued warning letters to two online marketers of semaglutide (Wegovy/Ozempic) and tirzepatide (Mounjaro) for selling unapproved, untested drugs to consumers through their websites. The two companies, US Chemlabs and Synthetix, have an opportunity to make proper adjustments, according to the letters. Failure to adequately address the concerns within 15 business days could result in legal action from FDA.
Other purveyors of knock-off semaglutide are being shut down and Novo Nordisk announced settlements with two clinic/spa facilities being forced to cease selling unapproved versions of semaglutide products. Novo brought legal action against two Florida clinics — Nuvida Rx Weight Loss in Tallahassee and Cosmetic Laser Professionals Med Spa in Miami — for “trademark infringement, false advertising, and unfair competition, according to both settlements.
Read More: Novo Nordisk Warns of Wegovy Supply Limitations & the Rise of Counterfeit Ozempic
Novo Nordisk Lawsuits
The increased demand for the semaglutide-based Wegovy and Ozempic has led to supply limitations and counterfeit products showing up at med spas and clinics. The company filed legal actions against multiple medical spas, weight loss or wellness clinics and compounding pharmacies to cease false advertising, trademark infringement and/or unlawful sales of non-FDA-approved compounded products claiming to contain semaglutide.
The lawsuits alleged that counterfeit compounded semaglutide Ozempic products were sold as genuine Novo Nordisk products, posing consumer confusion and potential safety concerns. The claims in the lawsuits are unrelated to the actual safety or effectiveness of Novo Nordisk's products.
Novo Nordisk is the only company in the United States with FDA-approved products containing semaglutide, and no FDA-approved generic versions of semaglutide currently exist. Ozempic and Rybelsus have FDA approvals to treat Type II diabetes, while Wegovy is FDA-approved to treat chronic weight management. It is also common for patients to use Ozempic off-label for weight loss.
Novo Nordisk launched a website to provide information about the responsible use of Novo Nordisk's FDA-approved semaglutide products, the differences between Novo Nordisk's FDA-approved products and other medicines being labeled as semaglutide, as well as tools on how to spot a counterfeit injectable product.
In 2017, Novo Nordisk agreed to pay a $1.1 million settlement over allegations that Novo Nordisk's drug Victoza was extensively promoted for uses not approved by the FDA, which violates the Insurance Frauds Prevention Action. The settlement stems from allegations in a whistleblower lawsuit filed by Peter Dastous, an Endocrinology Diabetes Care Specialist formerly employed by Novo Nordisk.
In 2022, Novo Nordisk reached a settlement with the U.S. Department of Justice (DoJ) for $6.3 million regarding allegations that the company violated U.S. law by selling injection needles to the government that it manufactured in non-designated countries. That same year, the company also settled a three-year-old shareholder lawsuit that targeted the company’s public communications about its insulin business.
The claims settled by the agreements were allegations only and there has been no determination of liability.
FDA Warning Letters
FDA's letters to US Chemlabs and Synthetix featured similar wording. In both letters, FDA expressed concern over the labeling of the knockoff semaglutide products.
US Chemlabs claims to offer “highly purified peptides at near wholesale” and also references sales of thymalin, which is used to treat immune dysfunction conditions. The company's semaglutide, tirzepatide and thymalin products are also misbranded under section 502(f) of the FD&C Act, 21 U.S.C. 352(f), in that their labeling fails to bear adequate directions for their intended use, according to the letter.
Synthetix’s FDA warning letter says the company markets the products “for ‘research use only’ and ‘not for human consumption’” but that “evidence obtained from your website establishes that your products are intended to be drugs for human use.” The company was also warned about its mislabeling of semaglutide and tirzepatide products.
The letter to both stated, "Despite statements on your product labeling marketing your products as 'research chemicals only' and 'not for human consumption,' evidence obtained from your website establishes that your products are intended to be drugs for human use. Your products are drugs as defined by section 201(g) of the FD&C Act 21, U.S.C. 321(g) because they are intended to prevent, treat, or cure disease conditions and/or affect the structure or function of the body."
In the letter to US Chemlabs, FDA mentioned that the company is marketing Thymalin to children, despite the drug lacking FDA evaluation.
The letter stated, "In addition, FDA is particularly concerned that you market your 'Thymalin' product for use in children. Your product has not been evaluated by FDA for safety, effectiveness, and quality. The use of untested drugs can have unpredictable and unintended consequences, especially in vulnerable populations such as children and infants who may be at greater risk for adverse reactions associated with certain drug products due to differences in the ability of children to absorb, metabolize, distribute, or excrete such drug products or their metabolites."
The letters from FDA were not intended to be an all-inclusive statement of violations and the responsibility lies with the companies to investigate and determine violations based on requirements of federal law and FDA regulations.
FDA requested a response with specific actions taken by the companies within 15 working days of receipt of the letter. Failure to comply could result in legal action.
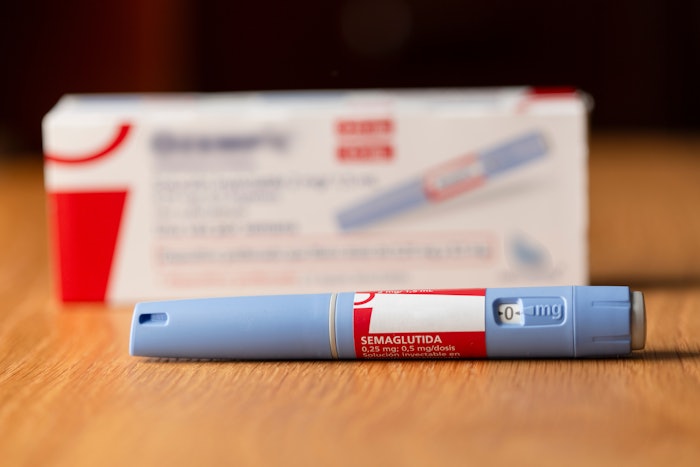
How to tell if it is Novo Nordisk Ozempic:
- Genuine Novo Nordisk Ozempic pens do not extend or increase in length when setting the dose.
- Authentic Ozempic pens are currently only available in a 0.25/0.5 mg pen, 1 mg pen or 2 mg pen.
- The box will include 4 needles that attach directly to the pen, except the Ozempic 0.25/0.5 mg dose carton, which has 6 needles.
- The dose dial window only shows intended doses:
- On the pen intended to deliver 0.25/0.5 mg doses, it only shows -0-, 0.25 and 0.5 once dialed up to the intended doses.
- On the pen intended to deliver a 1 mg dose, it only shows -0- and 1 mg once dialed up to the intended dose.
- On the pen intended to deliver a 2 mg dose, it only shows -0- and 2 mg once dialed up to the intended dose.
Signs of Counterfeit Ozempic:
- A counterfeit pen may be identified based on scale extending out from the pen when setting the dose.
- The label on a counterfeit pen could be of poor quality and may not adhere well to the pen.
- A counterfeit carton may have spelling mistakes on the front of the box (i.e., 1pen and 4 doses without space between ‘1’ and ‘pen’)
- A counterfeit carton may not include tamper-resistant/perforation.
- The batch number printed on a counterfeit box may not correspond to the product strength stated on the same box and pen.











